- Aïr
-
/ah"ear/, n.a region in N Niger, in the Sahara: low massif and oases. ab. 30,000 sq. mi. (77,700 sq. km). Also called Asben.
* * *
IMixture of gases constituting the earth's atmosphere.Some gases occur in steady concentrations. The most important are molecular nitrogen (N2), 78% by volume, and molecular oxygen (O2), 21%. Small amounts of argon (Ar; 1.9%), neon (Ne), helium (He), methane (CH4), krypton (Kr), hydrogen (H2), nitrous oxide (N2O), and xenon (Xe) are also present in almost constant proportions. Other gases occur in variable concentrations: water vapour (H2O), ozone (O3), carbon dioxide (CO2), sulfur dioxide (SO2), and nitrogen dioxide (NO2). Air also contains trace amounts of ammonia and hydrogen sulfide. The variable constituents are important for maintaining life. Water vapour is the source for all forms of precipitation and is an important absorber and emitter of infrared radiation. Carbon dioxide is necessary for photosynthesis and is also an important absorber and emitter of infrared radiation. Ozone in the stratosphere (see ozone layer) is an effective absorber of ultraviolet radiation from the Sun but at ground-level is a corrosive pollutant and a major constituent of smog.II(as used in expressions)Compagnie Internationale Air Franceair cushion vehicleEastern Air Lines Inc.* * *
▪ atmospheric gasmixture of gases comprising the Earth's atmosphere. The mixture contains a group of gases of nearly constant concentrations and a group with concentrations that are variable in both space and time. The atmospheric gases of steady concentration (and their proportions in percentage by volume) are as follows:nitrogen (N2) 78.084oxygen (O2) 20.946hydrogen (H2) 0.00005nitrous oxide (N2O) 0.00005See as table: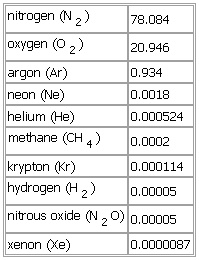 The uniformity of composition is maintained by mixing associated with atmospheric motions; but, above a height of about 90 km (55 miles), diffusional processes become more important than mixing, and the lighter gases (hydrogen and helium, in particular) are more abundant above that level.Of the gases present in variable concentrations, water vapour, ozone, carbon dioxide, sulfur dioxide, and nitrogen dioxide are of principal importance. The typical concentration ranges of these gases (in percentage by volume) are as follows:water vapour (H2O) 0 to 7ozone (O3) 0 to 0.01See as table:
The uniformity of composition is maintained by mixing associated with atmospheric motions; but, above a height of about 90 km (55 miles), diffusional processes become more important than mixing, and the lighter gases (hydrogen and helium, in particular) are more abundant above that level.Of the gases present in variable concentrations, water vapour, ozone, carbon dioxide, sulfur dioxide, and nitrogen dioxide are of principal importance. The typical concentration ranges of these gases (in percentage by volume) are as follows:water vapour (H2O) 0 to 7ozone (O3) 0 to 0.01See as table: Although present in relatively small amounts, these variable constituents may be very important for maintaining life on Earth's surface. Water vapour is the source for all forms of precipitation and is an important absorber and emitter of infrared radiation. carbon dioxide, besides being involved in the process of photosynthesis, is also an important absorber and emitter of infrared radiation. Ozone, which is present mainly in the atmospheric region 10 to 50 km (6 to 30 miles) above the Earth's surface, is an effective absorber of ultraviolet radiation from the Sun and effectively shields the Earth from all radiation of wavelengths less than 3,000 angstroms.
Although present in relatively small amounts, these variable constituents may be very important for maintaining life on Earth's surface. Water vapour is the source for all forms of precipitation and is an important absorber and emitter of infrared radiation. carbon dioxide, besides being involved in the process of photosynthesis, is also an important absorber and emitter of infrared radiation. Ozone, which is present mainly in the atmospheric region 10 to 50 km (6 to 30 miles) above the Earth's surface, is an effective absorber of ultraviolet radiation from the Sun and effectively shields the Earth from all radiation of wavelengths less than 3,000 angstroms.* * *
Universalium. 2010.
