- Abundances of elements
-
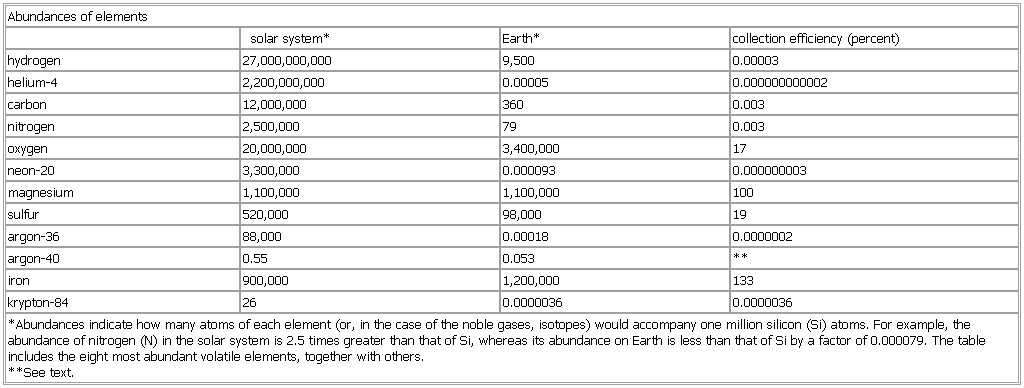
▪ TableAbundances of elementssolar system* Earth* collection efficiency (percent)hydrogen 27,000,000,000 9,500 0.00003helium-4 2,200,000,000 0.00005 0.000000000002carbon 12,000,000 360 0.003nitrogen 2,500,000 79 0.003oxygen 20,000,000 3,400,000 17neon-20 3,300,000 0.000093 0.000000003magnesium 1,100,000 1,100,000 100sulfur 520,000 98,000 19argon-36 88,000 0.00018 0.0000002argon-40 0.55 0.053 **iron 900,000 1,200,000 133krypton-84 26 0.0000036 0.0000036*Abundances indicate how many atoms of each element (or, in the case of the noble gases, isotopes) would accompany one million silicon (Si) atoms. For example, the abundance of nitrogen (N) in the solar system is 2.5 times greater than that of Si, whereas its abundance on Earth is less than that of Si by a factor of 0.000079. The table includes the eight most abundant volatile elements, together with others.**See text.See as table:
* * *
Universalium. 2010.
