Common ions that form multiple cations
- Common ions that form multiple cations
-
Common ions that form multiple cations
ion systematic name alternate name
Cu b; copper(I) cuprous
Co
3 b; cobalt(
III) cobaltic
Co
2 b; cobalt(
II) cobaltous
Hg
2 b; mercury(
II) mercuric
Hg22 b;(*) mercury(I) mercurous
*Mercury(I) ions always occur bound together to form Hg22 b;.
See as table: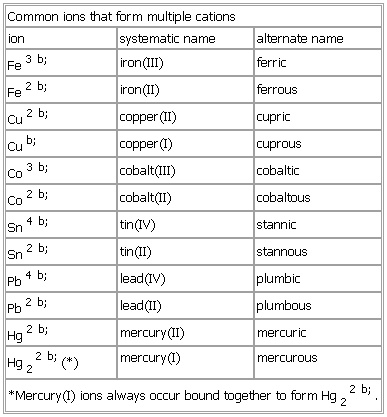
* * *
Universalium.
2010.
Look at other dictionaries:
chemical compound — Introduction any substance composed of identical molecules consisting of atoms (atom) of two or more chemical elements (chemical element). All the matter in the universe is composed of the atoms of more than 100 different chemical elements … Universalium
Metal ions in aqueous solution — A metal ion in aqueous solution is a cation, dissolved in water, of chemical formula [M(H2O)n]z+. The solvation number, n, determined by a variety of experimental methods is 4 for Li+ and Be2+ and 6 for elements in rows 3 and 4 of the periodic… … Wikipedia
chemical bonding — ▪ chemistry Introduction any of the interactions that account for the association of atoms into molecules, ions, crystals, and other stable species that make up the familiar substances of the everyday world. When atoms approach one another … Universalium
nervous system — Anat., Zool. 1. the system of nerves and nerve centers in an animal or human, including the brain, spinal cord, nerves, and ganglia. 2. a particular part of this system. Cf. autonomic nervous system, central nervous system, peripheral nervous… … Universalium
Action potential — In physiology, an action potential is a short lasting event in which the electrical membrane potential of a cell rapidly rises and falls, following a consistent trajectory. Action potentials occur in several types of animal cells, called… … Wikipedia
Nucleic acid tertiary structure — Example of a large catalytic RNA. The self splicing group II intron from Oceanobacillus iheyensis.[1] The tertiary structure of a nucleic acid is its precise three dimensional structure, as defined by the atomic coordinates.[2] … Wikipedia
Ion — Cation and Anion redirect here. For the particle physics/quantum computing concept, see Anyon. For other uses, see Ion (disambiguation). Hydrogen atom (center) contains a single proton and a single electron. Removal of the electron gives a cation … Wikipedia
Battery (electricity) — For other uses, see Battery (disambiguation). Various cells and batteries (top left to bottom right): two AA, one D, one handheld ham radio battery, two 9 volt (PP3), two AAA, one C, one … Wikipedia
cell — cell1 cell like, adj. /sel/, n. 1. a small room, as in a convent or prison. 2. any of various small compartments or bounded areas forming part of a whole. 3. a small group acting as a unit within a larger organization: a local cell of the… … Universalium
crystal — crystallike, adj. /kris tl/, n., adj., v., crystaled, crystaling or (esp. Brit.) crystalled, crystalling. n. 1. a clear, transparent mineral or glass resembling ice. 2. the transparent form of crystallized quartz. 3. Chem., Mineral. a solid body… … Universalium

