- Straight-chain saturated acids and their methyl esters
-
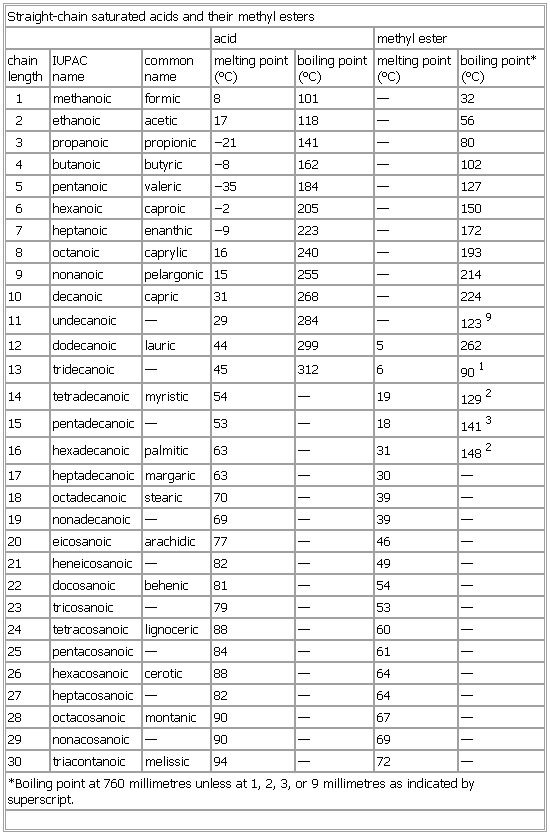
▪ TableStraight-chain saturated acids and their methyl estersacid methyl esterchainlength IUPACname commonname melting point(°C) boiling point(°C) melting point(°C) boiling point*(°C)1 methanoic formic 8 101 — 322 ethanoic acetic 17 118 — 563 propanoic propionic −21 141 — 804 butanoic butyric −8 162 — 1025 pentanoic valeric −35 184 — 1276 hexanoic caproic −2 205 — 1507 heptanoic enanthic −9 223 — 1728 octanoic caprylic 16 240 — 1939 nonanoic pelargonic 15 255 — 21410 decanoic capric 31 268 — 22411 undecanoic — 29 284 — 123912 dodecanoic lauric 44 299 5 26213 tridecanoic — 45 312 6 90114 tetradecanoic myristic 54 — 19 129215 pentadecanoic — 53 — 18 141316 hexadecanoic palmitic 63 — 31 148217 heptadecanoic margaric 63 — 30 —18 octadecanoic stearic 70 — 39 —19 nonadecanoic — 69 — 39 —20 eicosanoic arachidic 77 — 46 —21 heneicosanoic — 82 — 49 —22 docosanoic behenic 81 — 54 —23 tricosanoic — 79 — 53 —24 tetracosanoic lignoceric 88 — 60 —25 pentacosanoic — 84 — 61 —26 hexacosanoic cerotic 88 — 64 —27 heptacosanoic — 82 — 64 —28 octacosanoic montanic 90 — 67 —29 nonacosanoic — 90 — 69 —30 triacontanoic melissic 94 — 72 —*Boiling point at 760 millimetres unless at 1, 2, 3, or 9 millimetres as indicated by superscript.See as table:
* * *
Universalium. 2010.
