Coordination numbers and geometries of metal cyanide complexes
- Coordination numbers and geometries of metal cyanide complexes
-
Coordination numbers and geometries of metal cyanide complexes
electron
configuration* metal ion cyanide
complex geometry total number of
valence electrons
d2 Mo
4+ [Mo(
CN)
8]
4− dodecahedral 18
d3 Cr
3+ [Cr(
CN)
6]
3− octahedral 15
d4 Mn
3+ [Mn(
CN)
6]
3− octahedral 16
d5 Fe
3+ [Fe(
CN)
6]
3− octahedral 17
d6 Co
3+ [Co(
CN)
6]
3− octahedral 18
d7 Co
2+ [Co(
CN)
5]
3− square pyramidal 17
d8 Ni
2+ [Ni(
CN)
4]
2− square planar 16
d8 Ni
2+ [Ni(
CN)
5]
3− square pyramidal or
trigonal bipyramidal 18
d10 Cd
2+ [Cd(
CN)
4]
2− tetrahedral 18
d10 Ag
+ [Ag(
CN)
2]
− linear 14
*Number of d electrons indicated by superscript.
See as table: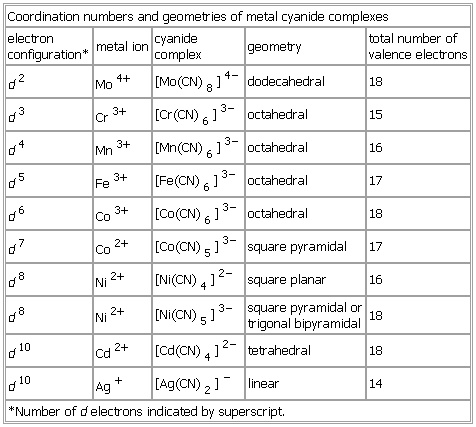
* * *
Universalium.
2010.
Look at other dictionaries:
coordination compound — Chem. complex (def. 10). Also called coordination complex. * * * ▪ chemistry Introduction any of a class of substances with chemical structures in which a central metal atom is surrounded by nonmetal atoms or groups of atoms, called ligands… … Universalium
Coordination complex — Cisplatin, PtCl2(NH3)2 A platinum atom with four ligands In chemistry, a coordination complex or metal complex, is an atom or ion (usually metallic), bonded to a surrounding array of molecules or anions, that are in turn known as ligands or… … Wikipedia
Complex (chemistry) — The term complex in chemistry is usually used to describe molecules or ensembles formed by the combination of ligands and metal ions. Originally, a complex implied a reversible association of molecules, atoms, or ions through weak chemical bonds … Wikipedia

