- pKa's of representative acids and bases
-
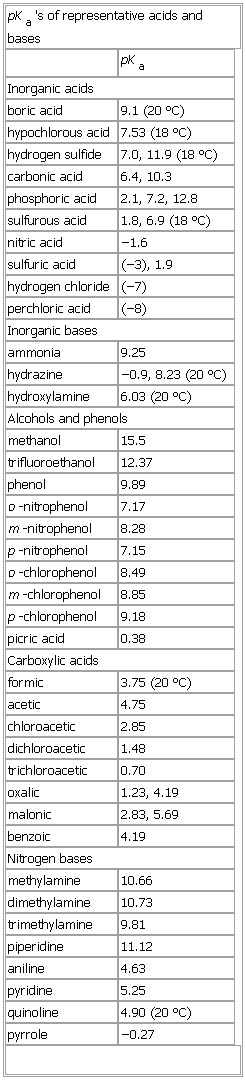
▪ TablepKa's of representative acids and basespKaInorganic acidsboric acid 9.1 (20 °C)hypochlorous acid 7.53 (18 °C)hydrogen sulfide 7.0, 11.9 (18 °C)carbonic acid 6.4, 10.3phosphoric acid 2.1, 7.2, 12.8sulfurous acid 1.8, 6.9 (18 °C)nitric acid −1.6sulfuric acid (−3), 1.9hydrogen chloride (−7)perchloric acid (−8)Inorganic basesammonia 9.25hydrazine −0.9, 8.23 (20 °C)hydroxylamine 6.03 (20 °C)Alcohols and phenolsmethanol 15.5trifluoroethanol 12.37phenol 9.89o-nitrophenol 7.17m-nitrophenol 8.28p-nitrophenol 7.15o-chlorophenol 8.49m-chlorophenol 8.85p-chlorophenol 9.18picric acid 0.38Carboxylic acidsformic 3.75 (20 °C)acetic 4.75chloroacetic 2.85dichloroacetic 1.48trichloroacetic 0.70oxalic 1.23, 4.19malonic 2.83, 5.69benzoic 4.19Nitrogen basesmethylamine 10.66dimethylamine 10.73trimethylamine 9.81piperidine 11.12aniline 4.63pyridine 5.25quinoline 4.90 (20 °C)pyrrole −0.27See as table:
* * *
Universalium. 2010.
